For the past while now, Kaleigh has been ghost writing our website News entries. I only say “ghost writing” because we couldn’t figure out how to add her name to the entries. Well you are now (temporarily) back to me (Amy) because Kaleigh has taken a job as a medical writer with Fingerpaint Group! Science writing has always been an interest of hers, and she even did an internship with UPMC media relations. Kaleigh has a couple of publications that we are wrapping up, and one was just published this week. This manuscript is the completion of a major part of her thesis work. This was a TON of fantastic work! Here is her write up of the study:
*undisclosed location
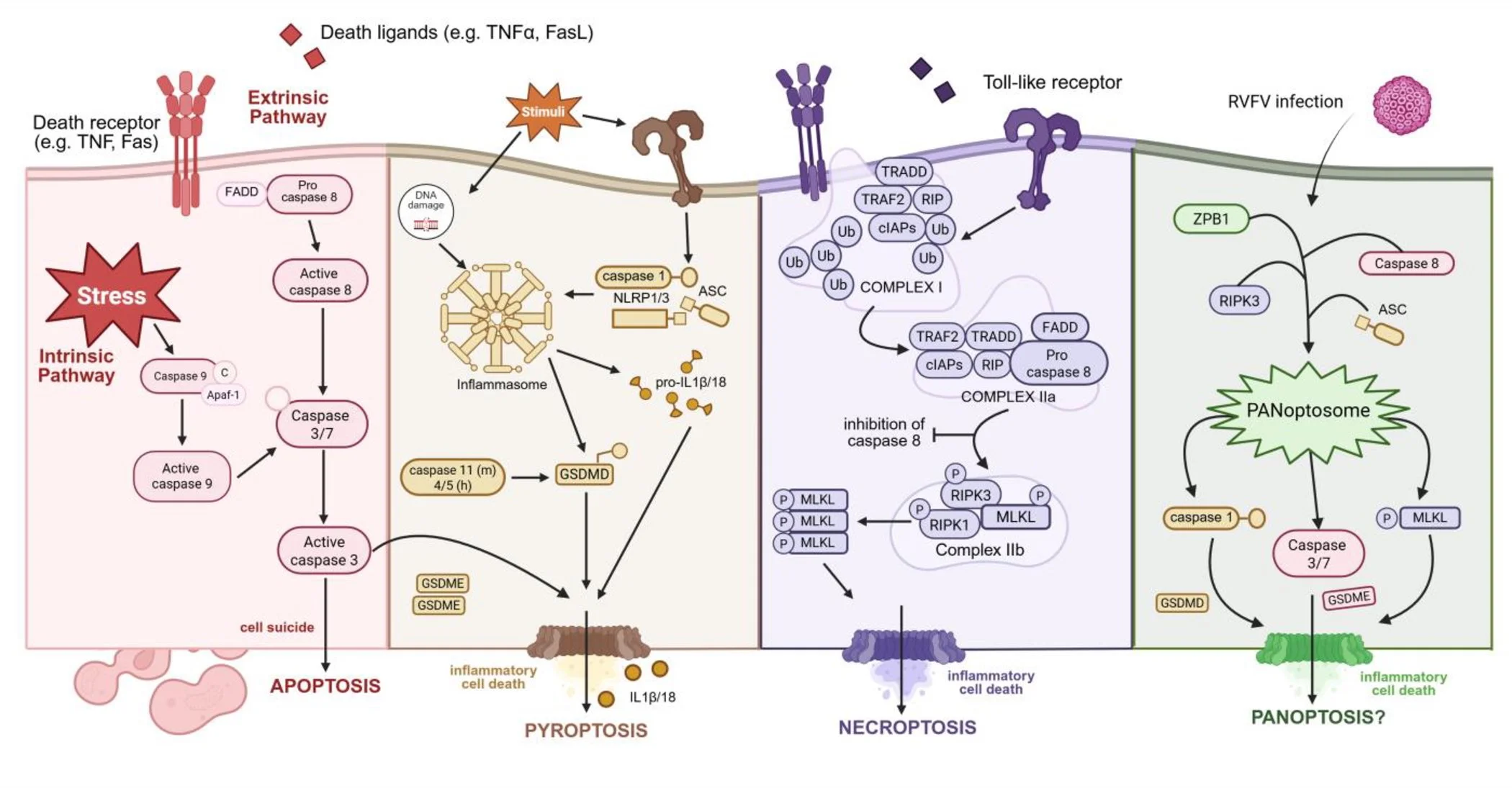
I’m so excited to share that one of the final chapters of my dissertation work is out now in the Journal of Virology! We demonstrate that Rift Valley fever virus (RVFV) results in the activation of multiple cell death pathways in neurons – a critical and fragile target of viral infection in the brain!
Using both wildtype and attenuated strains of RVFV, we used immunoblotting and fluorescent microscopy to describe which pathways of cell death were activated in primary neurons during infection. The answer: several!
• Using whole brains obtained at endpoint in a RVFV encephalitis model, we show the activation of proteins associated with apoptosis, necroptosis, and pyroptosis. This supports findings by our group and others demonstrating the susceptibility of neural cells to RVFV infection.
• Next, moving into a primary model using rat cortical neurons, we demonstrate that RVFV infection by both wildtype (strain ZH501) and an attenuated strain (RVFV-delNSsNSm) results in robust viral replication and subsequent neuronal damage and death.
• We confirm the formation of activated caspase 3 filaments in the nuclei of RVFV-infected neurons that can be detected using immunofluorescent microscopy, even at 6 hpi. This aligns with the detection of RVFV-NSs filaments at the same timepoints.
• At 6, 12 and 24 hpi we demonstrate activation of proteins associated with apoptosis, necroptosis, and pyroptosis following infection with wildtype and attenuated RVFV, including cleaved caspase 3, gasdermin E, and phosphorylated-MLKL.
• To add to these results, we used a technique called In Cell Western (ICW) to perform more high-throughput analysis of protein activation. Compared to mock-infected neurons, we observed an increase in proteins associated with apoptosis, necroptosis, and pyroptosis.
• Our final goal was to see if we could promote neuron survival, and reduce RVFV infection, using cell death inhibitors. We tested pan-caspase inhibitor Z-VAD-FMK and caspase 3 inhibitor Z-DEVD-FMK against wildtype RVFV and found that both reduced the activation of caspase 3 in a dose-dependent manner. However, there was not impact on viral replication at 24 or 48 hpi.
Collectively, these findings show that in primary rat neurons, infection with RVFV activates multiple mechanisms of cell death - even in the presence of viral interference - highlighting the redundancy and cross-talk of these mechanisms. This study adds to our understanding of RVFV neurologic disease, and lays the groundwork for the development of targeted therapeutics that may be both neuroprotective and antiviral.
I am immensely proud of this work (which began in 2021!), and grateful to all of my co-authors and colleagues who have seen this project through. Hartman lab members Zach Frey and Matt Demers, my co-neurovirologists; Reed lab members Morgan Midgett, Connor Wiliams and Doug Reed supported the animal work; Zak Wills from the Department of Neurobiology who provided resources, techniques, and invaluable insight into this project; and of course my mentor Amy Hartman, without whom I would’ve never dove into RVFV infection in neurons!
Read the full article here: https://doi.org/10.1128/jvi.01742-25
Kaleigh
Figure 1 - RVFV & PAN-optosis pathways